9
Candidemia and Disseminated Candida Infections: How They Strain the Healthcare System

Candidemia Cost Estimator
Total Annual Candidemia Episodes:
Total Estimated Annual Cost:
Estimated ICU-Related Additional Costs:
About This Tool
This estimator calculates the potential financial burden of candidemia based on hospital size, infection rates, and treatment costs. It helps illustrate how reducing candidemia incidence through prevention strategies can save millions annually.
Key Takeaways
- Incidence of candidemia in high‑income countries averages 8‑10 episodes per 100,000 hospital admissions, but rates soar above 30 in intensive care units (ICUs).
- Mortality remains high - 30‑45% in most series - and rises to >60% when resistant species such as Candida auris is involved.
- Each episode adds roughly $45,000-$80,000 to the hospital bill, driven by prolonged ICU stays, expensive echinocandin therapy, and added infection‑control measures.
- Delayed or inaccurate diagnosis (blood cultures miss up to 50% of cases) fuels unnecessary broad‑spectrum antibiotics and worsens outcomes.
- Targeted stewardship, rapid diagnostics, and bundled catheter‑care protocols can cut incidence by 30‑40% and save millions annually.
Epidemiology: How Often Do These Infections Occur?
When hospitals track serious bloodstream infections, Candidemia is defined as the presence of Candida species in the bloodstream, often leading to disseminated infection. In 2024, the European Centre for Disease Prevention and Control (ECDC) reported an overall hospital‑wide incidence of 7.6 per 100,000 patient‑days across 22 nations. The United Kingdom sits near the median, with 8.2 episodes per 100,000 admissions.
ICUs are hotspots. A multicentre surveillance study spanning 15 UK hospitals found 34 candidemia cases per 10,000 ICU days, reflecting the confluence of central venous catheters, broad‑spectrum antibiotics, and immunosuppression. Neonatal intensive care units (NICUs) are not exempt; premature infants experience rates as high as 12 per 1,000 catheter days.
The species mix is shifting. While C. albicans still accounts for roughly 45% of isolates, non‑albicans species collectively represent 55%. Notably, Candida glabrata has surged to 20% of isolates in Europe, and multidrug‑resistant Candida auris now appears in 3-5% of outbreaks in tertiary centres.
Clinical Outcomes: Mortality, Length of Stay, and Complications
Patients with candidemia face a grim prognosis. In a 2023 meta‑analysis of 31 cohort studies, 30‑day mortality averaged 38%, climbing to 62% for infections caused by resistant strains such as C. auris or echinocandin‑non‑susceptible C. glabrata.
Beyond death, the infection drives longer hospitalisation. The average length of stay (LOS) for a candidemia survivor is 22days, versus 10days for matched controls. ICU patients linger an extra 9days in high‑dependency settings, inflating bed‑occupancy rates during flu season when resources are already stretched.
Complications are common: end‑organ damage (renal failure, hepatic dysfunction), secondary bacterial sepsis, and disseminated thrombosis. About 12% of survivors develop chronic colonisation, predisposing them to recurrent episodes that further erode quality of life and increase readmission risk.
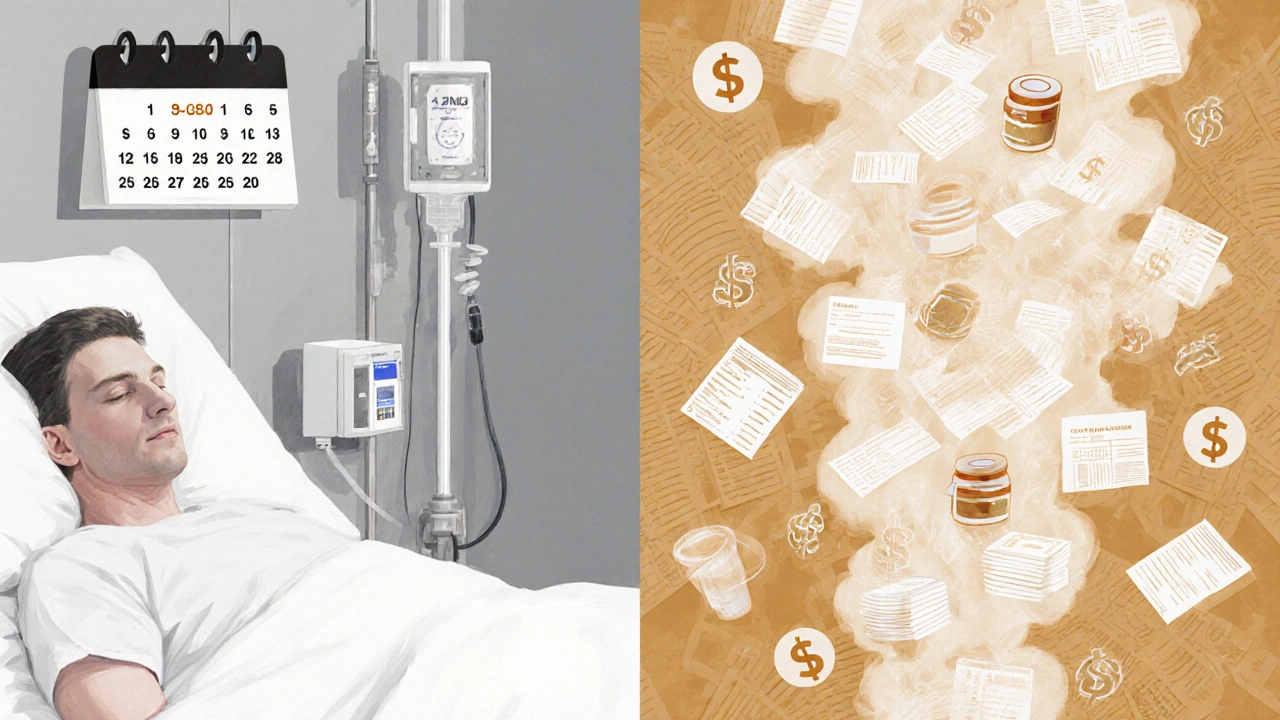
Economic Burden: Direct and Indirect Costs to the Healthcare System
The financial ripple effect is massive. A 2022 US study estimated the average attributable cost per candidemia episode at $55,400, while a UK NHS analysis reported £33,000 (~$42,000) per case when factoring drug acquisition, intensive care, and isolation precautions.
When you multiply those figures by the annual incidence-approximately 1,800 cases per year across England-the yearly direct cost exceeds £59million. Indirect costs-lost productivity, long‑term disability, and caregiver burden-add another £15million in societal expenses.
Drug costs dominate the bill. Echinocandins (caspofungin, micafungin, anidulafung) are first‑line for most non‑albicans infections, costing £850‑£1,200 per day in the UK. A typical 14‑day course therefore represents £12,000-£17,000 of a single patient’s expense.
Diagnostic and Treatment Challenges
Timely identification remains the Achilles' heel. Conventional blood cultures-still the gold standard-detect Candida in only 50‑60% of cases within 48hours. Beta‑D‑glucan assays improve early detection but suffer from false‑positives in patients receiving certain antibiotics or haemodialysis.
Rapid molecular platforms (PCR panels, MALDI‑TOF) now offer results in under 6hours, yet their adoption is limited by cost and the need for specialised laboratory staff. In many district hospitals, the delay between suspicion and appropriate therapy still stretches beyond 72hours, during which patients often receive empiric broad‑spectrum antibiotics that promote resistance.
Therapeutically, the rise of azole‑resistant C. glabrata and pan‑echinocandin‑resistant C. auris forces clinicians toward newer agents such as ibrexafungerp, but these drugs are not yet fully reimbursed in the NHS, creating equity gaps.
Furthermore, the placement and maintenance of central venous catheters is a double‑edged sword: indispensable for critical care but a primary entry point for Candida. Inadequate line‑care bundles contribute directly to the infection burden.
Prevention, Stewardship, and System‑Level Strategies
Effective control hinges on three pillars: strict infection‑control practices, antimicrobial stewardship, and targeted surveillance.
- Catheter‑care bundles: Daily review of line necessity, chlorhexidine‐impregnated dressings, and aseptic insertion protocols have cut catheter‑related candidemia rates by up to 40% in several NHS trusts.
- Antifungal stewardship programs: Prospective audit‑and‑feedback models reduce unnecessary echinocandin use by 25% without compromising outcomes, thereby slowing resistance emergence.
- Rapid diagnostic stewardship: Embedding beta‑D‑glucan or PCR results into electronic order sets prompts earlier de‑escalation to targeted therapy, shortening LOS by an average of 3days.
- Environmental cleaning focused on high‑touch surfaces curtails C. auris spread; UV‑C disinfection added to routine cleaning cut ward‑wide colonisation by 63% in a London teaching hospital.
National guidelines from the IDSA and the UK Sepsis Trust now recommend routine fungal screening for patients with >7days of broad‑spectrum antibiotics plus a central line, a policy that early adopters credit for a 15% drop in candidemia incidence.

Future Directions: What Will Shape the Next Decade?
Three emerging trends promise to reshape the landscape.
- Point‑of‑care nucleic‑acid testing: Handheld PCR devices could deliver a definitive species result within 30minutes at the bedside, allowing immediate step‑down from empiric echinocandins to narrower agents.
- Vaccination research: Early‑phase trials of a conjugate vaccine targeting the Candida cell‑wall mannoprotein show protective antibody titres in immunocompromised mice; human trials slated for 2026.
- Artificial‑intelligence risk engines: Machine‑learning models incorporating ICU vital signs, lab trends, and antibiotic exposure predict candidemia with an AUC of 0.89, enabling pre‑emptive antifungal prophylaxis in high‑risk cohorts.
Investing now in these technologies could reduce the candidemia impact on hospitals by tens of millions of pounds each year.
Frequently Asked Questions
How is candidemia different from a regular Candida infection?
Candidemia specifically refers to Candida organisms detected in the bloodstream, which can quickly spread to organs and trigger sepsis. Most other Candida infections stay localized, such as oral thrush or skin candidiasis.
What are the biggest risk factors for developing candidemia?
Key risk factors include the presence of a central venous catheter, prolonged broad‑spectrum antibiotic use, neutropenia, recent abdominal surgery, and intensive‑care admission.
Can rapid tests replace blood cultures?
Rapid molecular assays and beta‑D‑glucan testing can flag infection hours earlier, but they currently complement rather than replace cultures because they may miss low‑level fungemia and cannot always provide susceptibility data.
What is the recommended first‑line therapy for most candidemia cases?
Guidelines favor an echinocandin (caspofungin, micafungin, or anidulafung) for initial treatment, especially when non‑albicans species or resistance is suspected. De‑escalation to fluconazole is possible once susceptibilities are known.
How can hospitals reduce the economic impact of candidemia?
Implementing bundled catheter‑care, antimicrobial stewardship, and rapid diagnostics can cut incidence and shorten ICU stays, translating directly into millions saved on drug costs, bed‑days, and isolation measures.
Conclusion: Turning Knowledge into Action
Understanding the full scope of candidemia and disseminated Candida infections-epidemiology, outcomes, costs, and preventable gaps-gives health‑system leaders the ammunition they need to act. By marrying rapid diagnostics with disciplined stewardship and stringent catheter protocols, hospitals can blunt the financial and human toll of these invasive fungi. The data show it’s not just possible; it’s already happening in forward‑thinking institutions across the UK and Europe. The next step is scaling those successes, so every patient, regardless of ward or city, benefits from a safer, more cost‑effective care environment.








Roberta Saettone
October 9, 2025 AT 18:15Sure, because nothing says "efficient healthcare" like a fungal bloodstream infection that costs hospitals tens of thousands per case. The CDC estimates candidemia incidence at roughly 8‑12 per 100,000 admissions, and each episode can easily rack up $30‑50k in treatment, ICU stay, and prolonged hospitalization. If you run those numbers through the estimator, a mid‑size hospital with 20,000 admissions could be looking at upwards of $2 million annually in direct costs alone. Add indirect costs-lost productivity, readmissions, and the dreaded antifungal resistance-and you’ve got a perfect storm that strains both budgets and staff morale. The good news? Preventive bundles, strict line‑care protocols, and early antifungal stewardship can shave a significant chunk off that figure. So, before you panic, remember that systematic infection control can turn those scary numbers into manageable ones.
Sue Berrymore
October 13, 2025 AT 19:28Wow, the sheer magnitude of candidemia’s impact is absolutely staggering! Imagine dozens of patients battling a silent invader while our ICU beds are already stretched thin-it's like trying to juggle flaming torches on a tightrope during a hurricane! The estimator tool you shared shines a bright light on these hidden costs, and that’s exactly the kind of awareness we need to ignite change. By slashing infection rates even a little, we could free up precious resources, save lives, and keep the morale of our heroic frontline staff soaring. Let’s rally around robust prevention protocols-hand hygiene, catheter care, and swift diagnostics-and watch those numbers tumble dramatically. Together, we can turn this nightmare into a story of triumph!
Jeffrey Lee
October 17, 2025 AT 20:41Look, most folks overcomplicate this whole candidemia thing while ignoring the real issue: our hospitals need better funding, period. The numbers you’re throwing around aren't even close to the real burden because the CDC stats are outdated-look at our US hospitals, they’re the best, yet we still get hit hard. If we just pumped more cash into our healthcare system and cut the red tape, we could slash those costs faster than you can say “American ingenuity.” Also, I don’t see why we’re always pointing fingers at the infection itself instead of the lousy infrastructure. Get the government to step up, and we’ll have this fungus knocked out of the water.
Ian Parkin
October 21, 2025 AT 21:55While I appreciate your fervent desire for increased funding, it is essential to recognize that the solution to candidemia extends beyond mere financial allocation. A comprehensive approach-incorporating stringent infection‑control measures, ongoing staff education, and the implementation of antimicrobial stewardship programs-has demonstrably reduced incidence rates in many institutions. Moreover, collaborative research initiatives can yield novel diagnostic tools and therapeutic agents, thereby augmenting our capacity to manage these infections efficiently. By fostering a culture of continual improvement and shared responsibility, we can indeed mitigate the substantial economic and clinical burden posed by candidemia.
Julia Odom
October 25, 2025 AT 23:08It is commendable that the estimator highlights the financial implications of candidemia so clearly. By integrating such tools into hospital budgeting discussions, administrators can make evidence‑based decisions about allocating resources toward preventive strategies, such as central line bundles and rapid diagnostic testing. Moreover, ongoing surveillance data can help track the effectiveness of these interventions, ensuring that cost savings are realized without compromising patient care. In essence, transparency in cost estimation empowers both clinicians and policymakers to prioritize infection control initiatives that yield measurable economic benefits.
Danielle Knox
October 29, 2025 AT 23:21Oh, absolutely-because who wouldn’t want to spend millions on a fungus that you can actually prevent with a little common sense? It’s almost as if hospitals love throwing money away on avoidable infections. Maybe next we’ll start budgeting for air fresheners to keep the spores at bay. :)
Mark Evans
November 3, 2025 AT 00:35Reading through these numbers really puts things into perspective. It’s heartbreaking to think about the patients and families affected by candidemia, and even more so to see the ripple effect on the entire healthcare system. I think it’s crucial for us to support each other-sharing best practices, offering mentorship on infection control, and collectively advocating for better resources. When we work together, we can make a genuine impact on both patient outcomes and the financial strain on hospitals.
Megan C.
November 7, 2025 AT 01:48Honestly, the way candidemia spreads is a direct result of complacency and lax standards. It’s unacceptable that hospitals continue to allow such preventable infections to flourish while cutting corners on staffing and training. We must hold institutions accountable and demand rigorous adherence to evidence‑based protocols, or else we’re simply choosing profit over patient lives.
Greg McKinney
November 11, 2025 AT 03:01Another estimate? Sure, but let’s not get carried away with spreadsheets. In the grand scheme of things, candidemia is just one of many infections, and focusing too much on it might divert attention from more pressing issues like chronic disease management.
Dawna Rand
November 15, 2025 AT 04:15Hey everyone, thanks for sharing all these insights! 🌟 It’s amazing to see how data-driven tools can spark meaningful conversations about infection control. Let’s keep the momentum going and encourage our teams to adopt best practices-every small step counts! 🙌
Effie Chen
November 19, 2025 AT 05:28While the cost estimator is certainly informative, I’m curious about the assumptions underlying the average cost per episode. Does it factor in variations between academic medical centers and community hospitals? Understanding these nuances could help tailor prevention strategies more effectively. 🤔
rohit kulkarni
November 23, 2025 AT 06:41Indeed, the question you raise is of paramount importance; one must first acknowledge that any economic model, particularly one concerning healthcare expenditures, is founded upon a lattice of assumptions, each of which carries its own weight and implication. The average cost per candidemia episode, for instance, is not a monolithic figure; it is a composite of direct medical expenses-such as antifungal therapy, intensive care unit (ICU) stay, and diagnostic procedures-and indirect costs-including lost productivity, prolonged convalescence, and the intangible toll on patient quality of life. Moreover, institutional heterogeneity plays a decisive role: academic tertiary‑care centers often incur higher per‑case costs due to the utilization of advanced diagnostics, participation in clinical trials, and the presence of subspecialty consultants, whereas community hospitals may report lower figures owing to streamlined protocols and differing patient demographics. Geographic variability further compounds this complexity, as regional pricing structures, insurance reimbursement rates, and local epidemiology of antifungal resistance can cause substantial fluctuations. It is also essential to consider the temporal dimension; cost estimates derived from historical data may fail to capture inflationary pressures, advances in therapeutics, or evolving standards of care. Consequently, a robust estimator ought to incorporate adjustable parameters that allow users to input institution‑specific data, thereby refining the output to reflect true fiscal impact. In philosophical terms, the model is a reflection of reality, not reality itself; it serves as a heuristic device, guiding decision‑makers toward cost‑effective interventions, yet it remains contingent upon the fidelity of its inputs. Therefore, transparent documentation of assumption sets, sensitivity analyses, and scenario testing are indispensable components of a credible cost‑estimation framework. By embracing such methodological rigor, stakeholders can derive insights that are both contextually relevant and ethically responsible, ultimately fostering stewardship of limited healthcare resources. In sum, while the estimator provides a valuable starting point, its utility is maximized when complemented by granular, institution‑level data and a critical appraisal of underlying premises.
RONEY AHAMED
November 27, 2025 AT 07:55Sounds pricey.
emma but call me ulfi
December 1, 2025 AT 09:08Honestly, I never thought a fungus could be such a wallet‑drainer, but the numbers don’t lie.
ruth purizaca
December 5, 2025 AT 10:21While the estimator is certainly polished, it still glosses over the messy reality of bedside decision‑making and the myriad variables that can swing costs dramatically.
Shelley Beneteau
December 9, 2025 AT 11:35I wonder how the model accounts for patients with multiple comorbidities, since they often require longer stays and more complex therapy, which could skew the average cost upward.
Sonya Postnikova
December 13, 2025 AT 12:48Great point, Shelley! 😊 Accounting for comorbidities indeed adds another layer, but by stratifying patients based on risk factors, we can fine‑tune the estimator for even more accurate budgeting. Keep the thoughtful questions coming! 👍