Tag: FDA post-market surveillance
Caroline Wagstaff
Jan
5
5
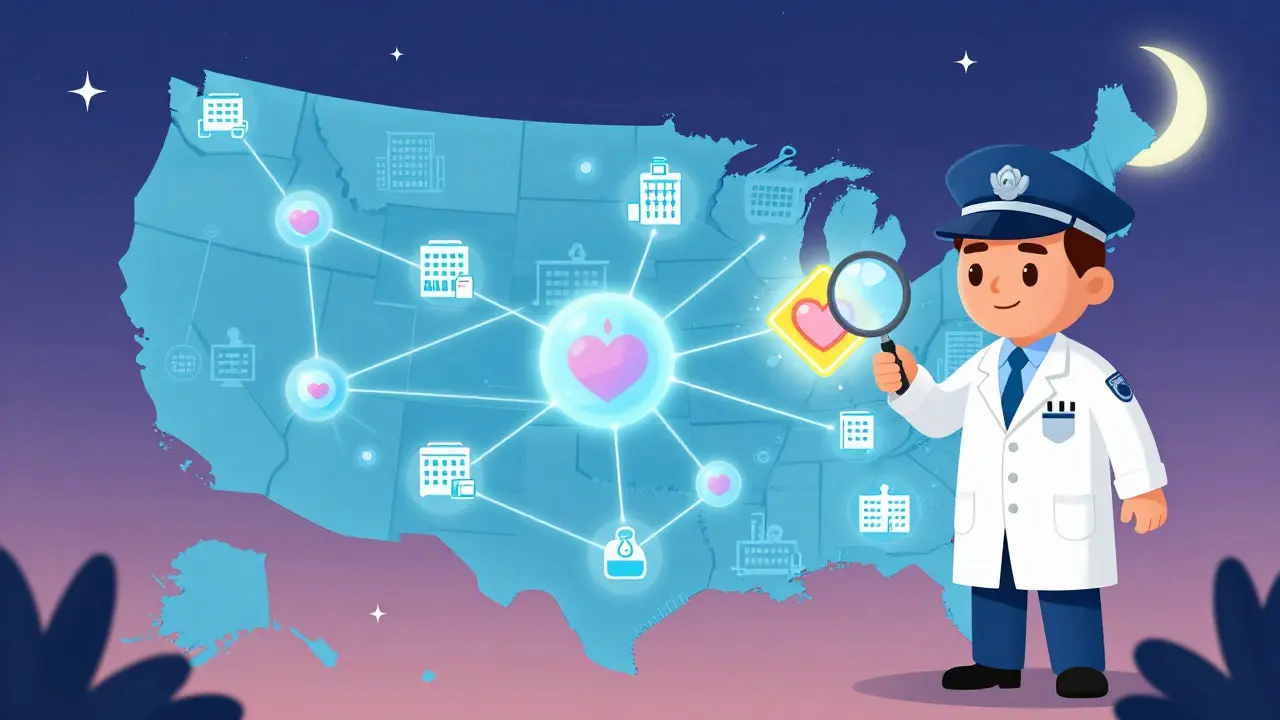
FDA Sentinel Initiative: How Big Data Detects Drug Safety Issues
The FDA Sentinel Initiative uses big data from millions of medical records to detect drug side effects in real time. Unlike passive reporting systems, it actively monitors real-world use to protect public health.
Latest Posts
Popular Posts
-
 Accidental Pediatric Medication Overdose: How to Prevent It and What to Do If It Happens
Accidental Pediatric Medication Overdose: How to Prevent It and What to Do If It Happens
-
 Out-of-Pocket Costs: How Generics Cut Your Drug Bills - and When They Still Hurt
Out-of-Pocket Costs: How Generics Cut Your Drug Bills - and When They Still Hurt
-
 Spinal Cord Injury: Understanding Function Loss, Rehabilitation, and Assistive Devices
Spinal Cord Injury: Understanding Function Loss, Rehabilitation, and Assistive Devices
-
 Duloxetine and Liver Health: What You Need to Know About Hepatotoxicity Risk
Duloxetine and Liver Health: What You Need to Know About Hepatotoxicity Risk
-
 Meniere’s Diet: How Sodium Restriction and Fluid Balance Reduce Vertigo and Hearing Loss
Meniere’s Diet: How Sodium Restriction and Fluid Balance Reduce Vertigo and Hearing Loss